Neurofibromatosis type 1 (NF1) is a rare, progressive, genetic condition characterized by benign tumors called plexiform neurofibromas (PN) that develop along nerve sheaths throughout the body, with signs and symptoms typically present at birth.1-4 Though benign, PN are highly variable and, depending on their size and location, have the potential to cause serious clinical complications, including pain, disfigurement, loss of function, spinal compression, and airway obstruction.5,6
The unpredictability and complexity of NF1 PN make this a particularly challenging disease to treat.1,5,6
NF1 PN
NF1 PN: A progressive disease that may have a big impact on patients
NF1 is caused by mutations in the NF1 gene that codes for neurofibromin, a tumor suppressor protein.6
-
Defective functioning of neurofibromin leads to a loss of suppression of RAS and unregulated cell proliferation, implicating tumor formation6-8
PN are one of the most prevalent tumors associated with NF1
-
NF1 occurs in 1 out of every 3000 individuals2
-
Up to 50% of children with NF1 have plexiform neurofibromas (PN) identified based on full-body MRI scan9,10
-
These tumors are highly variable in terms of size, shape, and growth rate, making it impossible to predict disease severity for any individual patient1,5
Because they can grow along any peripheral nerve in the body, PN have the potential to affect multiple organs, causing clinical complications.2,6
PN can become progressive
Plexiform neurofibromas typically manifest at birth, and some may grow rapidly during the first decade of life. These tumors can grow at any age and are unpredictable between PN in the same individual.4,11,12
The National Cancer Institute (NCI) conducted a long-term Natural History study of patients with NF1, which highlighted the progressive nature of plexiform neurofibromas and the development of PN-related morbidities.13
Out of 92 patients in the
Natural History study,
76% had
≥20% increase in PN volume
over a 2.8-year period 13
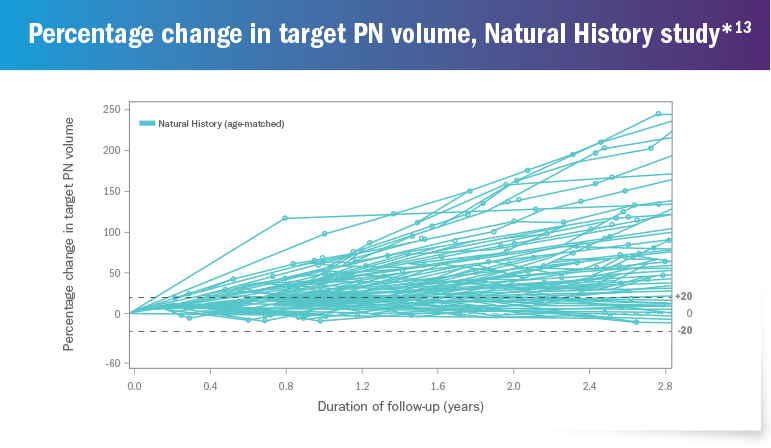
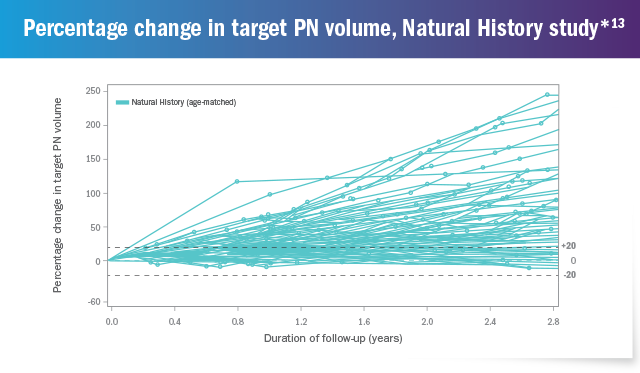
*The Natural History study began enrollment in 2008 and is ongoing. As of October 2018, 111 patients were enrolled who had NF1-related PN. 92 patients with NF1-related PN between the ages of 3 and 18 years who had at least 2 volumetric scans during the Natural History study are presented above.13
Of the 111 patients enrolled in the Natural History study by October 2018, a subset of 41 patients was selected for inclusion in a retrospective analysis based on the following eligibility criteria13,14:
-
Patients had comprehensive evaluations at least yearly until age 18 and thereafter at least every 3 years to characterize NF1-related manifestations
-
Patients were required to have at least one measurable PN, with MRI evaluation performed at 2 or more timepoints
-
PN-related symptoms (pain, motor, vision, bowel, bladder, and airway morbidities) were assessed
-
Patients had at least 7 years of clinical data
Consistent with previous studies, patients had PN that were widely distributed throughout the body14:
Conclusions from this retrospective subset analysis of 41 patients (with 57 PN) included14:
-
Median PN growth rate per year was 15.9%
-
86% of PN had >20% increase in tumor volume from baseline to maximum assessment
-
Median PN volume change between baseline and maximum assessments was 108.9%
-
>52% of PN had an increase in the number of associated functional morbidities between baseline and maximum PN volume assessment
-
The majority of PN had already resulted in morbidities at baseline (median age at baseline was 8 years) and even smaller tumors could result in morbidity depending on their location
Because PN can
be
progressive and often result in
increasing morbidities,
early evaluation
and intervention are critical 14,15
MRI=magnetic resonance imaging; RAS=rat sarcoma viral oncogene homolog.
CLINICAL COMPLICATIONS
NF1-related PN can lead to major clinical complications
Plexiform neurofibromas are typically present at birth.2 During a child’s early years, the only visible sign may be areas of discoloration or subtle soft-tissue overgrowth.5 Some plexiform neurofibromas may be located so deeply within the body that they go unnoticed until pain or other symptoms become present.5
However, diffuse plexiform tumors can rapidly grow to much larger sizes.5,16
One study (age range of patients: 2 to 76 years) found that nearly
77% of PN are invasive or displacing, meaning that they infiltrate multiple planes of tissue or put pressure
on nearby structures in the body, which can result in significant morbidity 17
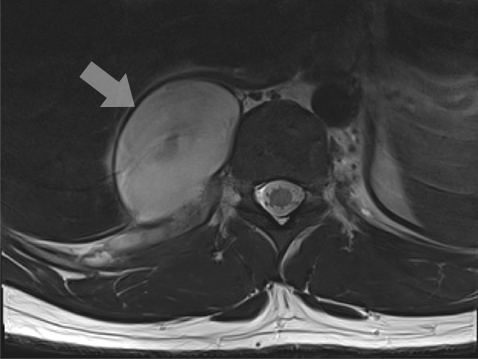
MRI of spinal plexiform neurofibromas.
Although benign, plexiform neurofibromas represent a major source of morbidity, with the potential to cause2,14:
Pain
Chronic PN-related pain may require ongoing
management with pain
medication*†18
Disfigurement
PN located in the head,
neck, and face can
result in
highly visible deformities5
Spinal cord compression
PN can compress nerves, resulting in pain,
weakness, numbness, and loss of motor
function,
as well as bladder or bowel dysfunction5
Vision impairment
When located near the orbital area,
PN can rarely result in visual impairmentǂ19
Airway compromise
PN that grow within the chest
can compromise breathing ability5
Even small tumors may cause
significant morbidity
depending
on their location 14
In the previously mentioned retrospective analysis of the NCI Natural History study, a subset of 41 patients was evaluated. Collectively, these patients reported 57 distinct PN (with some patients reporting multiple tumors). These PN were evaluated at baseline and at their individual maximum volume assessment. Conclusions from this analysis included14:
PN tended to develop early 20
-
70% of the PN evaluated had at least 1 associated morbidity at baseline (median baseline patient age was 8 years) 14
-
At baseline, the most common morbidity was pain (associated with 37% of PN)§ǁ14
-
Functional morbidities tended to increase over time 14
-
Between baseline and maximum PN volume assessment:
-
>52% of the PN evaluated had an increase in the number of associated functional morbidities 14
-
The number of PN with motor-related morbidities doubled from 11 to 22 14
-
8 PN, which had zero associated functional morbidities at baseline, developed 1 or more at the time of maximum assessment 14
-
Faster-growing PN were associated with increased use of pain medication 14
-
27 PN required an increase in the number of pain medications between baseline and maximum PN volume assessment 14
-
PN requiring an increase in pain medication generally grew faster than those PN that did not result in increased pain medication (median annual PN growth of 21% [n=27] vs 13% [n=30]) 14
PN are frequently
associated
with clinically significant
morbidities,
making early detection
and intervention
critical 14
*Children and adolescents with NF1 PN from 6 to 18 years of age, who were enrolled in the NCI Natural History study, were eligible for this pain sub-study of 60 patients. The aims of this study were to: 1. Assess the degree to which pain interferes with daily functioning (pain interference); 2. Describe the prevalence and type of pain medications used for treatment;
3. Examine the relationships between pain interference, disease factors, social-emotional functioning, and quality of life (QOL) in youth with NF1 PN. Caregivers and adolescents completed the Impact of Pediatric Illness (IPI) Scale, a general parent-proxy report and self-report QOL scale that assesses the effects of pediatric chronic illness on the domains of adaptive, emotional, physical, and cognitive functioning, including an item on pain interference.
†The limitations of this study include: 1. Pain interference was assessed using a single item from a general QOL scale for children with chronic illnesses. This question inquires only about the extent to which pain interferes with overall daily functioning and does not assess the impact of pain on specific activities or the frequency and intensity of pain, which would provide a more comprehensive assessment; 2. The response period of the scale was over the past month whereas measures assessing pain during a shorter time period (eg, in the past week or in real time) may provide more accurate data; 3. The study was cross-sectional; thus, the results of the mediational analyses are considered exploratory since longitudinal data are required to make more definitive statements of mediation; 4. The generalizability of the findings is limited by our specific patient population: the sample consisted only of youth with PN, many of whom were referred to the NCI for PN treatment trials and may have larger tumors and more severe complications than other children with NF1.
‡This is a retrospective, longitudinal case series of 37 patients (17 female) with orbitotemporal neurofibromatosis presented to the Australian Craniofacial Unit, Adelaide, between January 1981 and January 2009. Inclusion criteria were a diagnosis of NF1 according to National Institutes of Health diagnostic criteria with orbital or periorbital involvement.
§Based on a retrospective review of patients enrolled in the NCI NF1 Natural History study with ≥7 years of data available. Forty-one patients with 57 PN (min 1, max of 3 PN per patient) were included. Twenty-six patients were male and 15 were female. Median age at enrollment was 13 (min 5.2, max 31.3). Patients were followed at least yearly until age 18 and every 3 years thereafter to characterize NF1-related manifestations and their PN with volumetric MRI analysis.
||The limitations of this study include: 1. Due to many patients having their baseline MRI prior to enrollment in the NF1 Natural History study, the prospective evaluations of functional morbidity being used in that study could not be consistently utilized in the patient population, as these assessments were not available for the majority of baseline clinical assessments;
2. Sample size is still relatively small, and the significance of the findings may be limited by the multiple comparisons in this exploratory analysis.
MRI=magnetic resonance imaging.
TREATMENT CHALLENGES
NF1-related PN surgical challenges
Surgical removal may be an option and should be considered in the case of aesthetic disfigurement, pain, or functional deficits.1
However, the nature and structure of plexiform neurofibromas make complete resection challenging, and many of these tumors are deemed inoperable for reasons that include5:
Infiltrative quality
PN can be large and irregularly shaped, with a tendency to infiltrate beyond the nerve sheath into surrounding layers of tissue.5,6
-
Even partial removal can be difficult if the tumor has intertwined itself with vital tissue or nerves 5
Challenging locations
PN are often located in surgically challenging areas such as the head, neck, chest, or spine.5
-
PN located within the brachial plexus, the network of nerves that connect the arm and hands to the spinal cord, may be difficult to completely remove without sacrificing function or causing serious disability 5
High vascularity
PN tend to be overvascularized, which increases the risk of bleeding and the need for transfusion during surgery.5
-
Hematoma, a common complication of surgery, may require a temporary post-surgical drain 5
For patients with inoperable PN, treatment may focus on symptoms and complications as they occur and a careful “watch and wait” approach in which tumors are routinely reassessed.5,6
NF1 PN is a difficult-to-treat condition due to its unpredictability, progressive nature, and potential for serious clinical complications. 5,6 Managing NF1 is complex and requires a multidisciplinary approach. 5,20 A multidisciplinary team may help patients manage physical symptoms, including challenges that affect pediatric patients and their parents or caregivers. 5,21
Maintaining an open dialogue and engaging patients and caregivers throughout their journey can help them better manage treatment decisions as they cope with this debilitating condition. 5
NF1 with PNs:
A rare disease with enormous impact on patients and caregivers
Neurofibromatosis (NF) is a rare, progressive neurogenetic condition characterized by diverse and progressive manifestations that can include cutaneous, neurologic, skeletal, and neoplastic features3,4
- NF1 is caused by mutations in the NF1 gene that code for neurofibromin5
-
- Depletion of neurofibromin, a natural tumor suppressor, is associated with elevated RAS activity and cellular proliferation, which can result in the formation of tumors
- NF1 is typically diagnosed in early childhood and occurs in an estimated 1 out of every 3000 live births6-8
RAS=rat sarcoma viral oncogene homolog.
PNs are a significant challenge for patients with NF1
- 30% to 50% of patients with NF1 can develop PNs, which are tumors that grow along the nerve. Five to 10 percent of patients with NF1 develop malignant peripheral nerve sheath tumors (MPNSTs)7
- Many PNs are considered inoperable for reasons that include:
-
- Their complexity, invasive nature, and tendency to grow within and throughout the nerve sheath, all of which compromise the ability to achieve clear margins7,9
- Common locations, such as the head, neck, spine, and trunk, which may be surgically challenging1,9
- The risk of neurological damage and functional impairment as a result of surgery6
- The tumors’ vascularity and high propensity to bleed during surgery4,10
- NF1 with PNs is usually treated with a clinical team approach including geneticists, neurologists, oncologists, surgeons, as well as other health care professionals from relevant disciplines11,12
Consider the disease burden of NF1 with PNs
The pain, disfigurement, and functional impairment associated with NF1 has a significant impact on patients’ and caregivers’ quality of life and emotional well-being
- NF1 has a high burden of illness, particularly in patients with inoperable PNs2
- Individuals with NF1 are prone to developing both benign and malignant tumors of the CNS and peripheral nervous system2
- Tumors growing >20% per year have been shown to be significantly more frequent in children than adults13
- Patients may have better results when PNs are detected and addressed early14
CNS=central nervous system.
The pain that PNs inflict goes beyond physical impairment into psychosocial issues
- The lifelong consequences of NF1 include patients’ social alienation due to the visible nature of the disease and caregivers’ psychosocial and stress-related issues10
- The complex and unpredictable nature of the disease leads to a strong likelihood of psychological issues for patients, which can diminish their ability to experience a happy and fully engaged childhood6
NF1 with PNs can also emotionally impact caregivers
- Caregivers can experience significant emotional turmoil, including feelings of fear, anger, sadness, grief, and guilt2
- Symptoms of anxiety and depression are common for both patients and their caregivers, and psychiatrists and counselors can play an important role in managing psychological complications2
Current treatment for NF1 with PNs
Treatment for NF1 can be complex due to the lifelong impact of the disease, the need to surgically address the lesions, and the complications that may affect multiple organ systems2,11,15
- Surgical removal or debulking of PNs has been a mainstay of NF1 treatment; in some cases, surgical outcomes are favorable and lead to symptomatic improvement. Unfortunately, many PNs are deemed inoperable due to their2,11,15
-
- Size
- Location
- Involvement with nerves and organs
- For these patients, treatment may focus on symptomatic management and a careful monitoring approach in which tumors are routinely reassessed2
- Prognosis is poor for MPNSTs in patients with NF1. Treatment often requires off-label chemotherapy regimens that may include the use of doxorubicin and ifosfamide4,11
-
- There is currently no effective chemotherapy treatment regimen for PNs in NF12,7
- Currently there are no FDA-approved pharmacological treatments for PNs2,7
Therapy for other complications of NF1
- Treatment for mobility issues related to NF1 include surgery, supplements for bones (such as calcium and vitamin D), and lifestyle modifications (such as wearing a brace and/or receiving physical therapy)2,7
- Treatment for pain can include analgesics, including opioids and neuropathic pain remedies, and surgical debulking2,6
-
- Data suggest that 12% of children with PNs take narcotics and more than 70% of children and adults with NF1 use prescription pain medications16
- For NF1-related neurobehavioral issues such as cognitive deficits, patients may receive lovastatin and ADHD medications, while psychosocial treatments may involve antidepressants7
ADHD=Attention-deficit/hyperactivity disorder.
NF1 with PNs is a particularly difficult disease state due to its unpredictability, potential for disfigurement, and the emotional burden placed on children and their caregivers. Treatment is complex and requires a multidisciplinary approach to manage the physical symptoms and complications of PNs, as well as the psychosocial challenges.2,7
Maintaining an open dialogue, promptly discussing health-related changes, and engaging patients and caregivers as much as possible throughout their journey can help them cope with this rare and devastating condition.2
References:
1. Tonsgard JH. Clinical manifestations and management of neurofibromatosis type 1. Semin Pediatr Neurol. 2006;13(1):2-7. 2. Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13(8):834-843. 3. Blakeley JO, Plotkin SR. Therapeutic advances for the tumors associated with neurofibromatosis type 1, type 2, and schwannomatosis. Neuro Oncol. 2016;18(5):624-638. 4. Dombi E, Ardern-Holmes SL, Babovic-Vuksanovic D, et al. Recommendations for imaging tumor response in neurofibromatosis clinical trials. Neurology. 2013;81(21)(suppl 1):S33-S40. 5. Korf BR, Rubenstein AE. Neurofibromatosis: A Handbook for Patients, Families, and Health Care Professionals. Thieme Medical Publishers; 2005. 6. Hersh JH. Health supervision for children with neurofibromatosis. Pediatrics. 2008;121(3):633-642. 7. Yap YS, McPherson JR, Ong CK, et al. The NF1 gene revisited—from bench to bedside. Oncotarget. 2014;5(15):5873-5892. 8. Adil A, Singh A. Neurofibromatosis Type 1 (Von Recklinghausen). Updated June 3, 2019. Accessed August 16, 2022. https://www.ncbi.nlm.nih.gov/books/NBK459358/?report=printable 9. Nguyen R, Kluwe L, Fuensterer C, Kentsch M, Friedrich RE, Mautner VF. Plexiform neurofibromas in children with neurofibromatosis type 1: frequency and associated clinical deficits. J Pediatr. 2011;159(4):652-5.e2. 10. Miller DT, Freedenberg D, Schorry E, Ullrich NJ, Viskochil D, Korf BR. Health supervision for children with neurofibromatosis type 1. Pediatrics. 2019;143(5):e20190660. 11. Tucker T, Friedman JM, Friedrich RE, Wenzel R, Fünsterer C, Mautner VF. Longitudinal study of neurofibromatosis 1 associated plexiform neurofibromas. J Med Genet. 2009;46(2):81-85. 12. Nguyen R, Dombi E, Widemann BC, et al. Growth dynamics of plexiform neurofibromas: a retrospective cohort study of 201 patients with neurofibromatosis 1. Orphanet J Rare Dis. 2012;7:75. 13. Data on File, REF-75729, AstraZeneca Pharmaceuticals LP. 14. Gross AM, Singh G, Akshintala S, et al. Association of plexiform neurofibroma volume changes and development of clinical morbidities in neurofibromatosis 1. Neuro Oncol. 2018;20(12):1643-1651. 15. Friedrich RE, Schmelzle R, Hartmann M, Fünsterer C, Mautner VF. Resection of small plexiform neurofibromas in neurofibromatosis type 1 children. World J Surg Oncol. 2005;3(1):6. 16. Canavese F, Krajbich JI. Resection of plexiform neurofibromas in children with neurofibromatosis type 1. J Pediatr Orthop. 2011;31(3):303-311. 17. Mautner VF, Hartmann M, Kluwe L, Friedrich RE, Fünsterer C. MRI growth patterns of plexiform neurofibromas in patients with neurofibromatosis type 1. Neuroradiology. 2006;48(3):160-165. 18. Wolters PL, Burns KM, Martin S, et al. Pain interference in youth with neurofibromatosis type 1 and plexiform neurofibromas and relation to disease severity, social-emotional functioning, and quality of life. Am J Med Genet A. 2015;167A(9):2103-2113. 19. Greenwell TH, Anderson PJ, Madge SK, Selva D, David DJ. Long-term visual outcomes in patients with orbitotemporal neurofibromatosis. Clin Exp Ophthalmol. 2014;42(3):266-270. 20. Copley-Merriman C, Yang X, Juniper M, Amin S, Yoo HK, Sen SS. Natural history and disease burden of neurofibromatosis type 1 with plexiform neurofibromas: a systematic literature review. Adolesc Health Med Ther. 2021;12:55-66. 21. Ferner RE, Huson SM, Thomas N, et al. Guidelines for the diagnosis and management of individuals with neurofibromatosis 1. J Med Genet. 2007;44:81-88.


